Heat exchangers

Heat exchangers
Most chemical reactions are faster at higher temperatures and heat exchangers are frequently used to provide the heat necessary to increase the temperature of the reaction.
A common heat exchanger is the shell and tube type (Figures 12 and 13) where one part of the process flows through a tube and the other part around the shell.
A good example where heat exchange is important is in the manufacture of sulfur trioxide from sulfur dioxide in the Contact Process where the excess heat is used to warm incoming gases.
The heat from the reaction is transferred to incoming gases across the tube wall (Figure 12) and the rate of heat transfer is proportional to:
i) the temperature difference between the hot gases and the incoming gases and
ii) the total surface area of the tubes
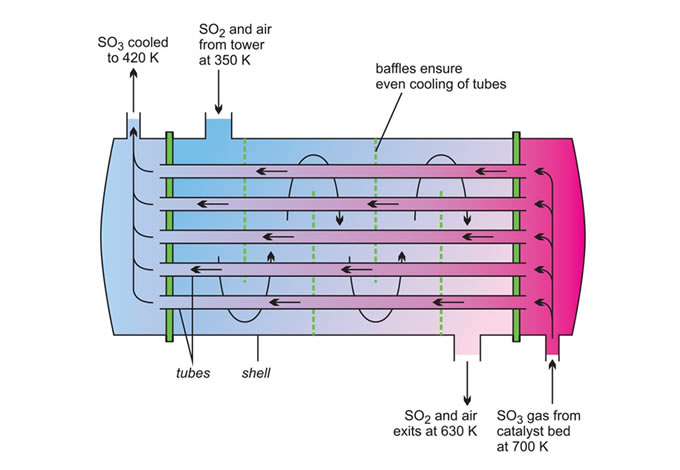
Thus the rate of heat transfer required will determine the size of the exchanger but when a chemical reaction also occurs in the exchanger (as in the case of tubular reactors ), it is important to take into account the residence time of the materials (whether they be gases or liquids) in the heat exchanger.
Another example of a heat exchanger is the condenser at the top of a distillation column. Here heat from the vapour emerging from the top of the column is removed by, for example, cooling water. The vapour cools down and condenses and the temperature of the water increases.
Some developments
In the future many chemicals may be produced in reactors about the size of a large desktop computer, known as microreactors. The reduced size will lead to a reduction in capital costs and a reduction in the amount of chemicals in use at any one time, resulting in an inherently safer process.
The temperature can be kept constant more readily (as there is a much larger surface for a given volume). This allows for a very efficient heat transfer from the reaction to the surroundings even for very exothermic reactions such as the nitration of an aromatic hydrocarbon which can potentially be explosive.
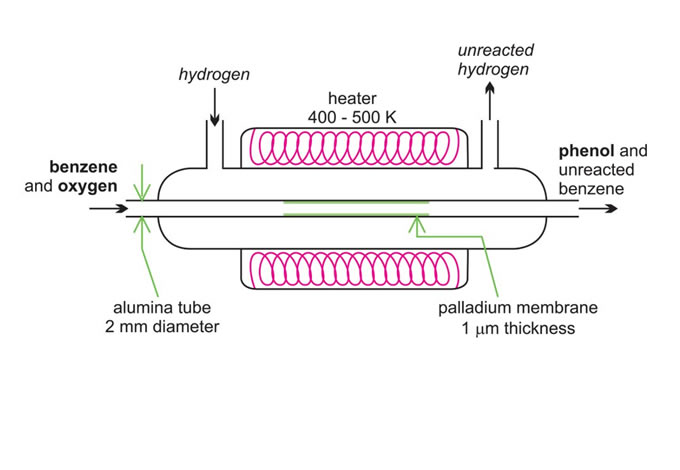
There is considerable amount of research being carried out in developing microreactors. One example is the possibility of the direct conversion of benzene to phenol. A mixture of benzene and oxygen is fed through an alumina tube, packed with palladium at ca 350-400 K and hydrogen gas is passed over it.
Hydrogen permeates through the alumina tube, and is converted to atomic hydrogen by the palladium catalyst. The hydrogen atoms react with oxygen, releasing reactive oxygen species, such as hydroxyl radicals, which in turn react with the benzene to form phenol.
Another development is known as oscillatory flow mixing. Chemical engineers are designing reactors where the fluids to be reacted are oscillated inside a reactor with baffles at frequencies between 0.5 and 15 Hz with amplitudes in the range 1 to 100 mm. This allows for very effective mixing of the reactants and also for heat to be transferred to the surroundings. This gives similar conditions to those in plug flow which are otherwise difficult to achieve with small quantities of materials.
